VisionArray ® Chip for HPV Genotyping
The VisionArray® HPV Chip 1.0 is intended to be used for the qualitative detection and genotyping of PCR-amplificates of 41 clinically relevant human papilloma virus (HPV) genotypes that have been produced with the help of the VisionArray® HPV PreCise Master Mix (Prod. No. ES-0007-50) from formalin-fixed, paraffin-embedded specimens, such as cervical carcinoma or head and neck squamous cell carcinoma. The chip is intended to be used in combination with a VisionArray® Software. The product is intended to be used as an aid to the differential diagnosis of cervical carcinoma or head and neck squamous cell carcinoma and therapeutic measures should not be initiated based on the test result alone.
VisionArray ® HPV Chip 1.0 - Detection of 41 HPV Types
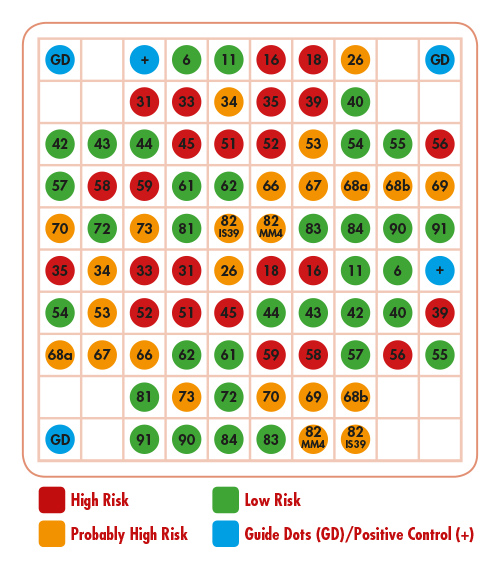
Chip Description
The components of the product are the chip as well as the VisionArray® HPV Chip File 1.0. Positioning of the capture sequences on the chip:
*HPV 55 is classified by now as subtype of HPV 44, but is still labeled HPV 55 for consistency reasons.
Ordering Information
|
Prod. No.: |
Tests: | Registration Status¹: |
|
VA-0001-10 |
10 |  |
| 1 |  In vitro diagnostic medical device according to EU directive 98/79/EC. In vitro diagnostic medical device according to EU directive 98/79/EC. CE IVD only available in certain countries. All other countries research use only! Please contact your local dealer for more information. |
Download Information
The VisionArray® HPV PreCise Master Mix is intended to be used to amplify and biotinylate specific sections of the L1 region of the Human Papilloma Virus (HPV) genomes by polymerase chain reaction (PCR). The VisionArray® HPV PreCise Master Mix is designed to amplify HPV types including but not limited to those detected by the corresponding VisionArray® HPV Chips and genomic sequences of the human HLA-DQA1 gene as a PCR positive control. The VisionArray® HPV PreCise Master Mix has to be used with the VisionArray® Detection Kit and the corresponding VisionArray® HPV Chips. The automated analysis has to be performed with a VisionArray® Software. The product is intended for professional use only. All tests using the above mentioned product should be performed in a certified, licensed anatomic pathology laboratory under the supervision of a pathologist/human geneticist by qualified personnel. The product is intended to be used as aid to the differential diagnosis of cervical carcinoma or head and neck squamous cell carcinoma and therapeutic measures should not be based on the test result alone.
Ordering Information
|
Prod. No.: |
Tests: | Registration Status¹: |
|
ES-0007-50 |
50 |  |
| 1 |  In vitro diagnostic medical device according to EU directive 98/79/EC. In vitro diagnostic medical device according to EU directive 98/79/EC. CE IVD only available in certain countries. All other countries research use only! Please contact your local dealer for more information. |
Download Information
VisionArray ® Detection Kit
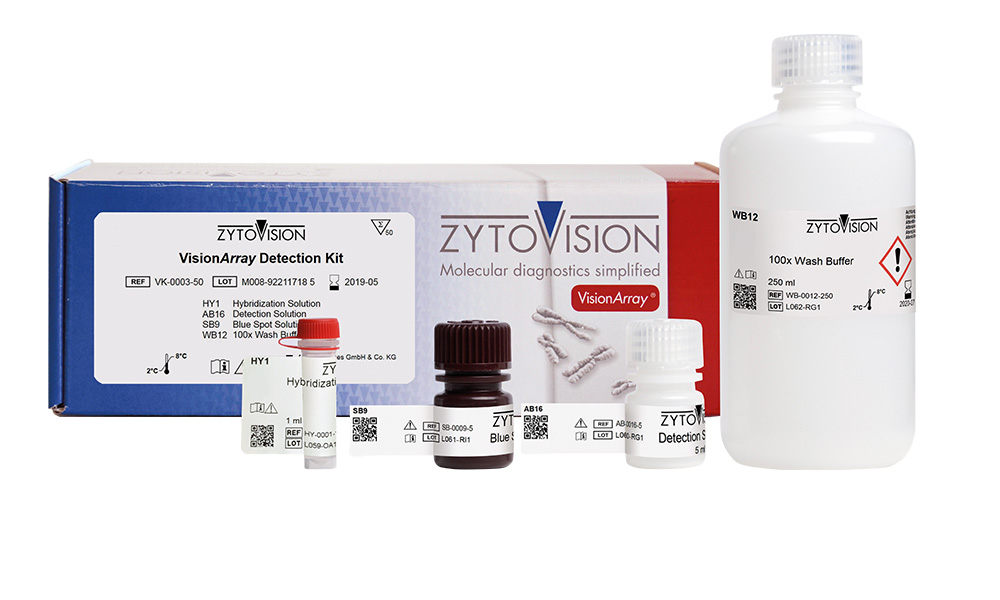
The VisionArray® Detection Kit is intended to be used with a VisionArray® PreCise Master Mix and the corresponding VisionArray® DNA Chip for the qualitative detection of specific DNA sequences. The automated analysis has to be performed with a VisionArray® Software. The product is intended for professional use only. All tests using the product should be performed in a certified, licensed anatomic pathology laboratory under the supervision of a pathologist/human geneticist by qualified personnel.
Ordering Information
|
Prod. No.: |
Tests: | Registration Status¹: |
|
VK-0003-50 |
50 |  |
| 1 |  In vitro diagnostic medical device according to IVDR (EU) 2017/746. In vitro diagnostic medical device according to IVDR (EU) 2017/746. CE IVD only available in certain countries. All other countries research use only! Please contact your local dealer for more information. |
Download Information
VisionArray ® Software
VisionArray ® MultiScan Software
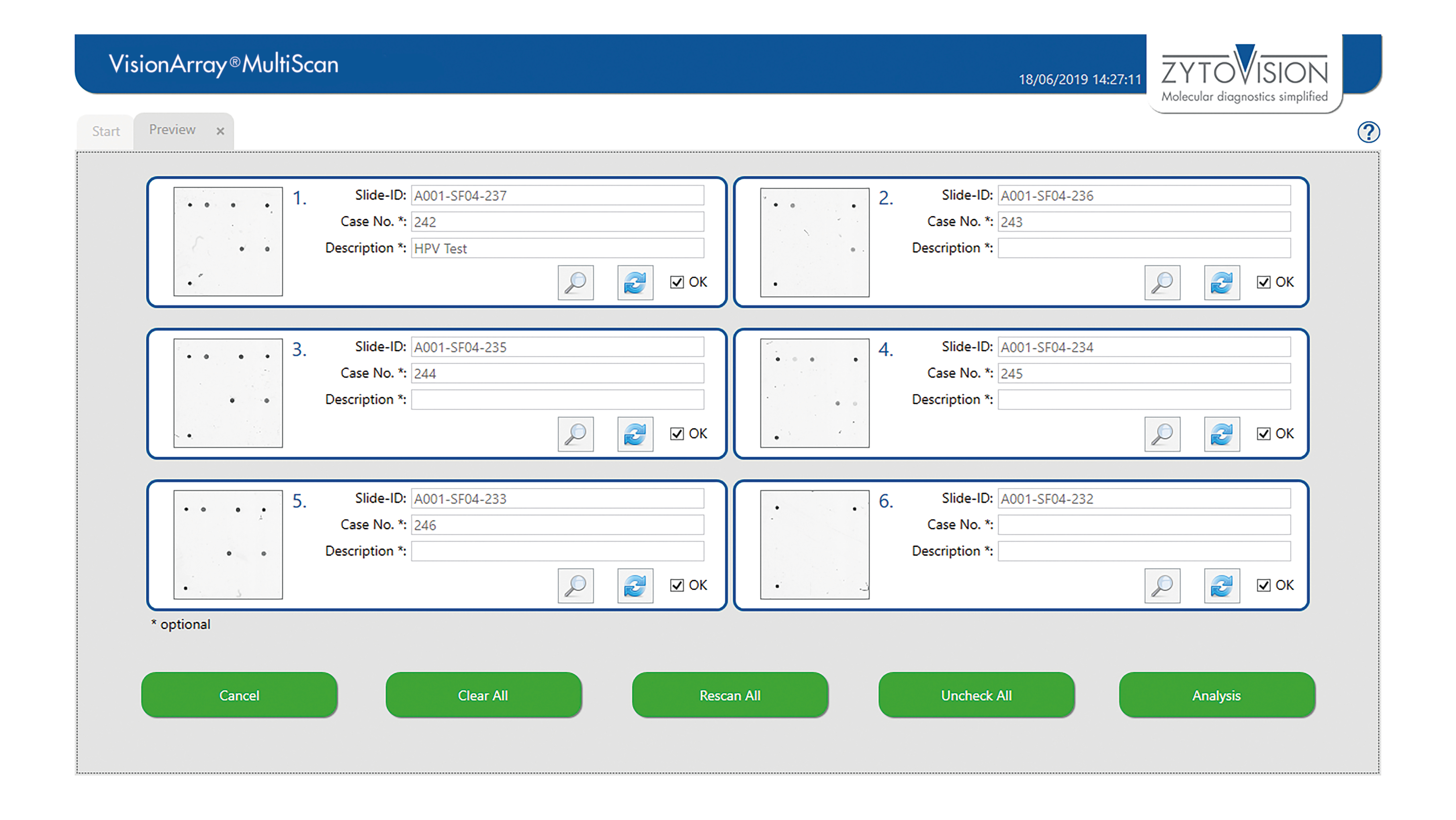
The VisionArray® MultiScan Software is intended to be used for the detection and analysis of hybridization signals on compatible microarray chips like VisionArray® Chips in combination with the corresponding chip file. The product is intended for professional use only. All tests using the product should be performed in a certified, licensed anatomic pathology laboratory under the supervision of a pathologist/human geneticist by qualified personnel.
For information on software installation services please contact your local dealer.
Ordering Information
|
Prod. No.: |
Registration Status¹: |
|
E-4302-1 |
 |
| 1 |  In vitro diagnostic medical device according to IVDR (EU) 2017/746. In vitro diagnostic medical device according to IVDR (EU) 2017/746. CE IVD only available in certain countries. All other countries research use only! Please contact your local dealer for more information. |
Download Information
VisionArray ® SingleScan Software
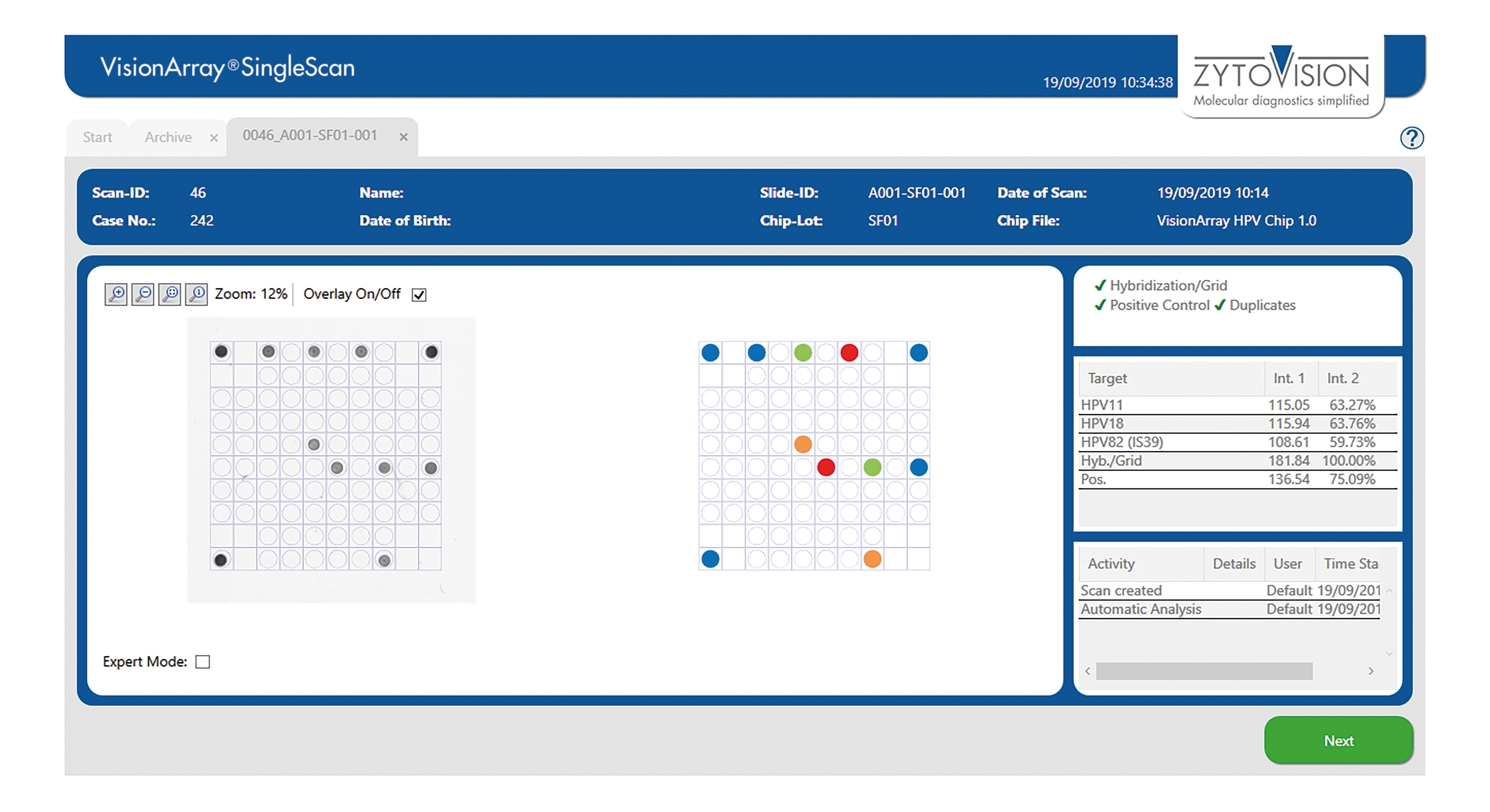
The VisionArray® SingleScan Software is intended to be used for the detection and analysis of hybridization signals on compatible microarray chips like VisionArray® Chips in combination with the corresponding chip file. The product is intended for professional use only. All tests using the product should be performed in a certified, licensed anatomic pathology laboratory under the supervision of a pathologist/human geneticist by qualified personnel.
For information on software installation services please contact your local dealer.
Ordering Information
|
Prod. No.: |
Registration Status¹: |
|
E-4301-1 |
 |
| 1 |  In vitro diagnostic medical device according to IVDR (EU) 2017/746. In vitro diagnostic medical device according to IVDR (EU) 2017/746. CE IVD only available in certain countries. All other countries research use only! Please contact your local dealer for more information. |


 PRODUCTS
PRODUCTS



















