VisionArray ® Chip for HPV Genotyping
The VisionArray® HPV Chip 1.0 is intended to be used for the qualitative detection and genotyping of PCR-amplificates of 41 clinically relevant human papilloma virus (HPV) genotypes that have been produced with the help of the VisionArray® HPV PreCise Master Mix (Prod. No. ES-0007-50) from formalin-fixed, paraffin-embedded specimens, such as cervical carcinoma or head and neck squamous cell carcinoma. The chip is intended to be used in combination with a VisionArray® Software. The product is intended to be used as an aid to the differential diagnosis of cervical carcinoma or head and neck squamous cell carcinoma and therapeutic measures should not be initiated based on the test result alone.
VisionArray ® HPV Chip 1.0 - Detection of 41 HPV Types
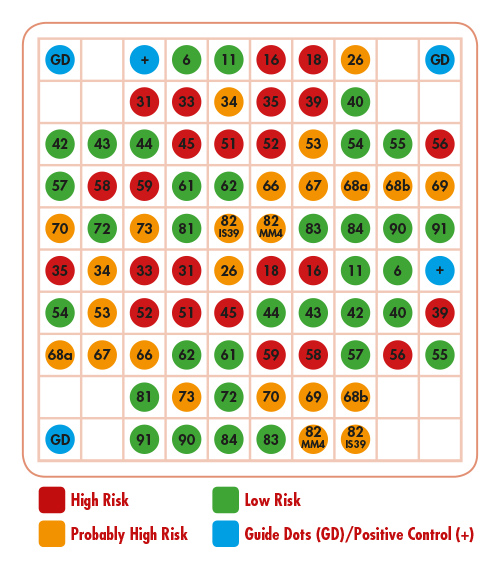
Chip Description
The components of the product are the chip as well as the VisionArray® HPV Chip File 1.0. Positioning of the capture sequences on the chip:
*HPV 55 is classified by now as subtype of HPV 44, but is still labeled HPV 55 for consistency reasons.
Ordering Information
|
Prod. No.: |
Tests: | Registration Status¹: |
|
VA-0001-10 |
10 |  |
| 1 |  In vitro diagnostic medical device according to EU directive 98/79/EC. In vitro diagnostic medical device according to EU directive 98/79/EC. CE IVD only available in certain countries. All other countries research use only! Please contact your local dealer for more information. |
Download Information
The VisionArray® HPV PreCise Master Mix is intended to be used to amplify and biotinylate specific sections of the L1 region of the Human Papilloma Virus (HPV) genomes by polymerase chain reaction (PCR). The VisionArray® HPV PreCise Master Mix is designed to amplify HPV types including but not limited to those detected by the corresponding VisionArray® HPV Chips and genomic sequences of the human HLA-DQA1 gene as a PCR positive control. The VisionArray® HPV PreCise Master Mix has to be used with the VisionArray® Detection Kit and the corresponding VisionArray® HPV Chips. The automated analysis has to be performed with a VisionArray® Software. The product is intended for professional use only. All tests using the above mentioned product should be performed in a certified, licensed anatomic pathology laboratory under the supervision of a pathologist/human geneticist by qualified personnel. The product is intended to be used as aid to the differential diagnosis of cervical carcinoma or head and neck squamous cell carcinoma and therapeutic measures should not be based on the test result alone.
Ordering Information
|
Prod. No.: |
Tests: | Registration Status¹: |
|
ES-0007-50 |
50 |  |
| 1 |  In vitro diagnostic medical device according to EU directive 98/79/EC. In vitro diagnostic medical device according to EU directive 98/79/EC. CE IVD only available in certain countries. All other countries research use only! Please contact your local dealer for more information. |
Download Information
VisionArray ® Detection Kit
The VisionArray® Detection Kit is intended to be used with a VisionArray® PreCise Master Mix and the corresponding VisionArray® DNA Chip for the qualitative detection of specific DNA sequences. The automated analysis has to be performed with a VisionArray® Software. The product is intended for professional use only. All tests using the product should be performed in a certified, licensed anatomic pathology laboratory under the supervision of a pathologist/human geneticist by qualified personnel.
Ordering Information
|
Prod. No.: |
Tests: | Registration Status¹: |
|
VK-0003-50 |
50 |  |
| 1 |  In vitro diagnostic medical device according to IVDR (EU) 2017/746. In vitro diagnostic medical device according to IVDR (EU) 2017/746. CE IVD only available in certain countries. All other countries research use only! Please contact your local dealer for more information. |
Download Information
VisionArray ® Software
VisionArray ® MultiScan Software
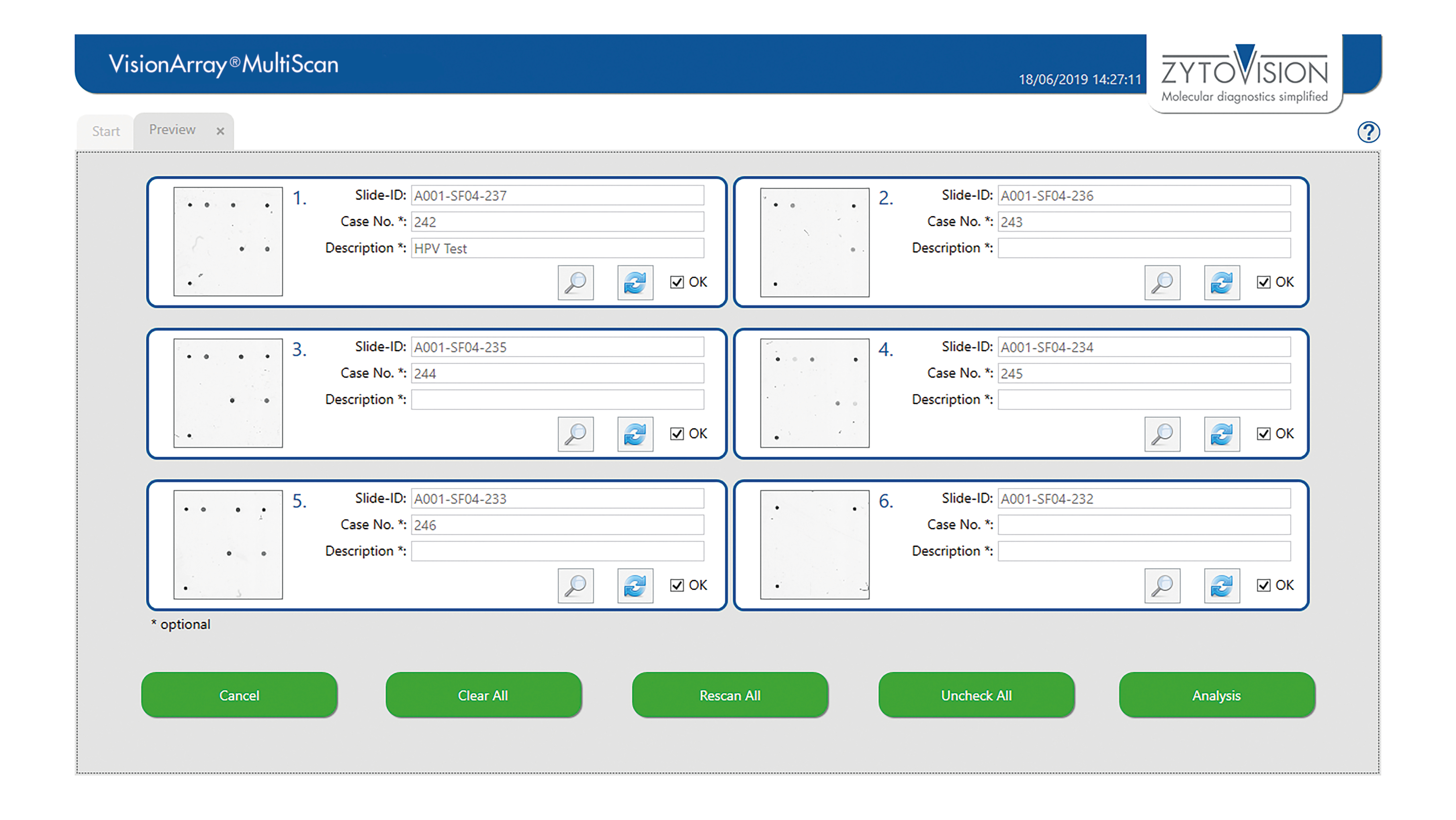
The VisionArray® MultiScan Software is intended to be used for the detection and analysis of hybridization signals on compatible microarray chips like VisionArray® Chips in combination with the corresponding chip file. The product is intended for professional use only. All tests using the product should be performed in a certified, licensed anatomic pathology laboratory under the supervision of a pathologist/human geneticist by qualified personnel.
For information on software installation services please contact your local dealer.
Ordering Information
|
Prod. No.: |
Registration Status¹: |
|
E-4302-1 |
 |
| 1 |  In vitro diagnostic medical device according to IVDR (EU) 2017/746. In vitro diagnostic medical device according to IVDR (EU) 2017/746. CE IVD only available in certain countries. All other countries research use only! Please contact your local dealer for more information. |
Download Information
VisionArray ® SingleScan Software
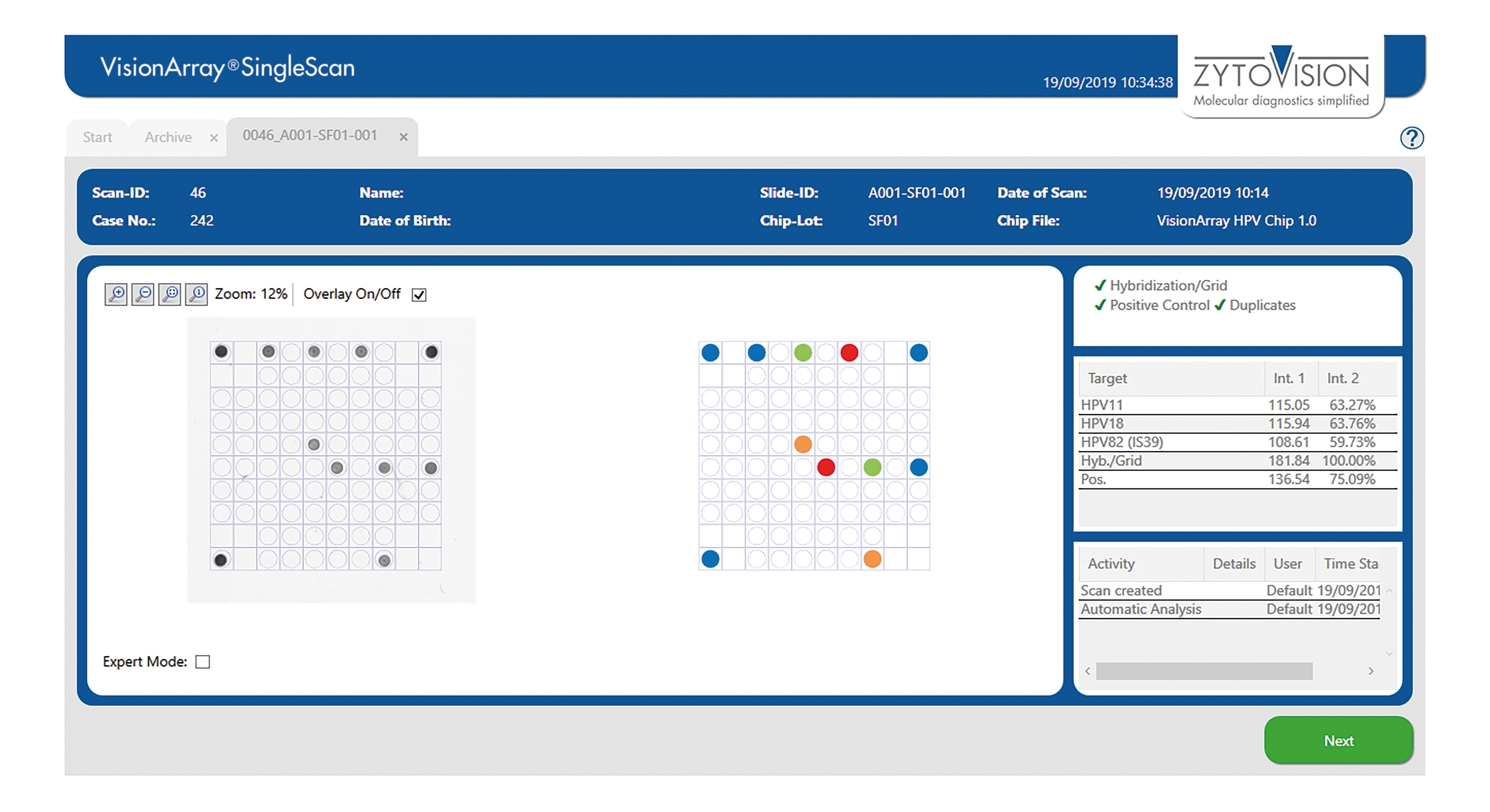
The VisionArray® SingleScan Software is intended to be used for the detection and analysis of hybridization signals on compatible microarray chips like VisionArray® Chips in combination with the corresponding chip file. The product is intended for professional use only. All tests using the product should be performed in a certified, licensed anatomic pathology laboratory under the supervision of a pathologist/human geneticist by qualified personnel.
For information on software installation services please contact your local dealer.
Ordering Information
|
Prod. No.: |
Registration Status¹: |
|
E-4301-1 |
 |
| 1 |  In vitro diagnostic medical device according to IVDR (EU) 2017/746. In vitro diagnostic medical device according to IVDR (EU) 2017/746. CE IVD only available in certain countries. All other countries research use only! Please contact your local dealer for more information. |
Download Information
VisionArray ® Chip for MYCO Genotyping
The mycobacterial genera comprise more than 140 species, which, for the purpose of diagnosis and treatment, have been grouped into three categories: M. tuberculosis complex (MTC), M. leprae, and non-tuberculous mycobacteria (NTM). The majority of the Mycobacterium species belongs to the NTM group and can be found in different environments. Many of these bacteria cause life-threatening infections in humans and in recent years, the mortality and morbidity associated with NTMs has increased especially in immunocompromised patients worldwide. Treatment of NTMs is specific to each species and therefore a clear distinction between the present species is of extreme importance. Reliable and rapid molecular diagnostics are the basis of an adequate therapy that is given by the VisionArray® MYCO Chip 2.0.
VisionArray ® MYCO Chip 2.0
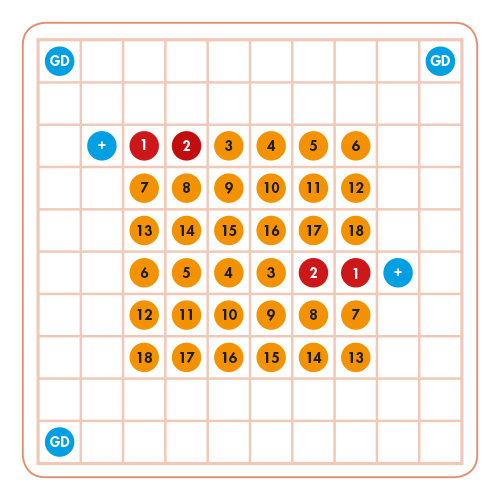
Chip Description
The VisionArray® MYCO Chip 2.0 is designed to detect several clinically relevant mycobacterial species. All capture sequences and the positive control are set up on the Chip as duplicates and the guide dots as triplicates. The signals are visible on the Chip as dark blue areas. The automated evaluation of the results is performed by a VisionArray® Software.
Ordering Information
|
Prod. No.: |
Tests: | Registration Status¹: |
|
VA-0005-10 |
10 |  |
| 1 |  In vitro diagnostic medical device according to EU directive 98/79/EC. In vitro diagnostic medical device according to EU directive 98/79/EC. CE IVD only available in certain countries. All other countries research use only! Please contact your local dealer for more information. |
Download Information
The VisionArray ® MYCO PreCise Master Mix 2.0 is intended to be used to amplify and biotinylate specific sections of the ITS and, in case of the M. tuberculosis complex, IS6110 region as well as the SR4 region (Zozaya-Valdés et al. 2017) of mycobacterial genomes by polymerase chain reaction (PCR) using DNA samples extracted from e.g. clinical specimens, pulmonary smears or cultivated samples. The VisionArray ® MYCO PreCise Master Mix 2.0 is designed to amplify mycobacteria including but not limited to those detected by the corresponding VisionArray ® MYCO Chips and, if present in the DNA sample, genomic sequences of the human HLA-DQA1 gene as a PCR positive control.
Ordering Information
|
Prod. No.: |
Tests: | Registration Status¹: |
|
ES-0008-50 |
50 |  |
| 1 |  In vitro diagnostic medical device according to EU directive 98/79/EC. In vitro diagnostic medical device according to EU directive 98/79/EC. CE IVD only available in certain countries. All other countries research use only! Please contact your local dealer for more information. |
Download Information
VisionArray ® Detection Kit

The VisionArray® Detection Kit is intended to be used with a VisionArray® PreCise Master Mix and the corresponding VisionArray® DNA Chip for the qualitative detection of specific DNA sequences. The automated analysis has to be performed with a VisionArray® Software. The product is intended for professional use only. All tests using the product should be performed in a certified, licensed anatomic pathology laboratory under the supervision of a pathologist/human geneticist by qualified personnel.
Ordering Information
|
Prod. No.: |
Tests: | Registration Status¹: |
|
VK-0003-50 |
50 |  |
| 1 |  In vitro diagnostic medical device according to IVDR (EU) 2017/746. In vitro diagnostic medical device according to IVDR (EU) 2017/746. CE IVD only available in certain countries. All other countries research use only! Please contact your local dealer for more information. |
Download Information
VisionArray ® Software
VisionArray ® MultiScan Software

The VisionArray® MultiScan Software is intended to be used for the detection and analysis of hybridization signals on compatible microarray chips like VisionArray® Chips in combination with the corresponding chip file. The product is intended for professional use only. All tests using the product should be performed in a certified, licensed anatomic pathology laboratory under the supervision of a pathologist/human geneticist by qualified personnel.
For information on software installation services please contact your local dealer.
Ordering Information
|
Prod. No.: |
Registration Status¹: |
|
E-4302-1 |
 |
| 1 |  In vitro diagnostic medical device according to IVDR (EU) 2017/746. In vitro diagnostic medical device according to IVDR (EU) 2017/746. CE IVD only available in certain countries. All other countries research use only! Please contact your local dealer for more information. |
Download Information
VisionArray ® SingleScan Software

The VisionArray® SingleScan Software is intended to be used for the detection and analysis of hybridization signals on compatible microarray chips like VisionArray® Chips in combination with the corresponding chip file. The product is intended for professional use only. All tests using the product should be performed in a certified, licensed anatomic pathology laboratory under the supervision of a pathologist/human geneticist by qualified personnel.
For information on software installation services please contact your local dealer.
Ordering Information
|
Prod. No.: |
Registration Status¹: |
|
E-4301-1 |
 |
| 1 |  In vitro diagnostic medical device according to IVDR (EU) 2017/746. In vitro diagnostic medical device according to IVDR (EU) 2017/746. CE IVD only available in certain countries. All other countries research use only! Please contact your local dealer for more information. |
Download Information
VisionArray ® – Array Based Detection of DNA Sequences
The VisionArray ® products are designed for the qualitative detection of specific DNA sequences by DNA/DNA hybridization on immobilized catcher molecules, which are arranged on a glass chip. All capture sequences and positive controls are set up on the VisionArray ® Chip as duplicates.
Advantages of VisionArray ®
- Quick & easy 1 hour protocol
- Automated evaluation using a VisionArray ® Analyzer Software – simple visualization & quick analysis in just a few minutes
VisionArray ® Chip for Genotyping of Pathogenic FUNGI
Fungal pathogens and infections are an increasing global public health concern. Recent assessments suggest that over a billion people are affected by fungal infections and more than 150 million people have serious fungal diseases, which have a major impact on their lives or are fatal. Mortality associated with fungal disease at >1.6 million per year is similar to that of tuberculosis. People most at risk are those with underlying health problems or a weakened immune system, such as chronic lung disease, prior tuberculosis, HIV, cancer, and diabetes mellitus. Early accurate diagnosis allows prompt antifungal therapy. However, this is often delayed or unavailable leading to death or serious chronic illness. The most frequently encountered pathogens are Aspergillus, Candida, Cryptococcus species, and Pneumocystis jirovecii, which are responsible for the majority cases of serious fungal disease. The diagnosis of invasive fungal infections is often challenging because of e.g. limited access to quality diagnostic tests. Direct microscopic examination of clinical samples may provide a tentative diagnosis, but this is often difficult to confirm by culture because of the presence of atypical fungal elements or sparse fungal populations. The VisionArray® Fungi Chip 1.0 is intended be used for the qualitative detection and genotyping of 30 clinically relevant fungi genotypes.
VisionArray ® FUNGI Chip 1.0
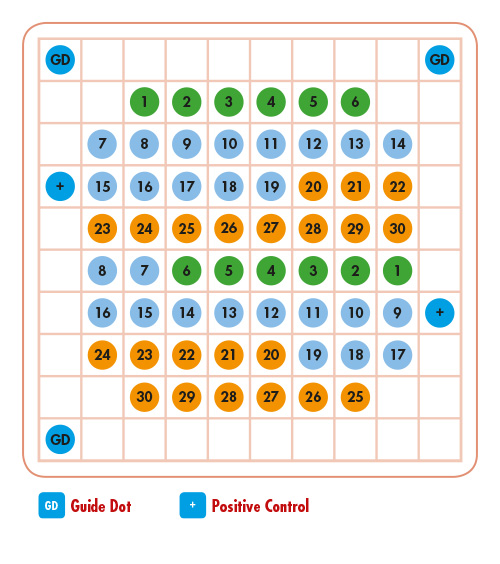
Chip Description
The VisionArray® FUNGI Chip 1.0 is intended to be used for the qualitative detection and genotyping of PCR-amplificates of 30 clinically relevant fungi genotypes that have been produced with the help of the VisionArray® FUNGI PreCise Master Mix 1.0 (Prod. No. ES-0009-50) from formalin-fixed, paraffin-embedded specimens. The chip is intended to be used in combination with a VisionArray® Software.
Ordering Information
|
Prod. No.: |
Tests: | Registration Status: |
|
VA-0006-10 |
10 |  |
Download Information
The VisionArray® FUNGI PreCise Master Mix 1.0 is intended to be used to amplify and biotinylate specific sections of the 16S-23S ITS region of fungi genomes by polymerase chain reaction (PCR). The VisionArray® FUNGI PreCise Master Mix 1.0 is designed to amplify fungi types including but not limited to those detected by the corresponding VisionArray® FUNGI Chips and genomic sequences of the human HLA-DQA1 gene as a PCR positive control.
Ordering Information
|
Prod. No.: |
Tests: | Registration Status: |
|
ES-0009-50 |
50 |  |
Download Information
VisionArray ® Detection Kit

The VisionArray® Detection Kit is intended to be used with a VisionArray® PreCise Master Mix and the corresponding VisionArray® DNA Chip for the qualitative detection of specific DNA sequences. The automated analysis has to be performed with a VisionArray® Software. The product is intended for professional use only. All tests using the product should be performed in a certified, licensed anatomic pathology laboratory under the supervision of a pathologist/human geneticist by qualified personnel.
Ordering Information
|
Prod. No.: |
Tests: | Registration Status: |
|
VK-0003-50 |
50 | When used in combination with VA-0006-10 and ES-0009-50, the whole test is then for research use only! |
Download Information
VisionArray ® Software
VisionArray ® MultiScan Software

The VisionArray® MultiScan Software is intended to be used for the detection and analysis of hybridization signals on compatible microarray chips like VisionArray® Chips in combination with the corresponding chip file. The product is intended for professional use only. All tests using the product should be performed in a certified, licensed anatomic pathology laboratory under the supervision of a pathologist/human geneticist by qualified personnel.
For information on software installation services please contact your local dealer.
Ordering Information
|
Prod. No.: |
Registration Status: |
|
E-4302-1 |
When used in combination with VA-0006-10 and ES-0009-50, the whole test is then for research use only! |
Download Information
VisionArray ® SingleScan Software

The VisionArray® SingleScan Software is intended to be used for the detection and analysis of hybridization signals on compatible microarray chips like VisionArray® Chips in combination with the corresponding chip file. The product is intended for professional use only. All tests using the product should be performed in a certified, licensed anatomic pathology laboratory under the supervision of a pathologist/human geneticist by qualified personnel.
For information on software installation services please contact your local dealer.
Ordering Information
|
Prod. No.: |
Registration Status: |
|
E-4301-1 |
When used in combination with VA-0006-10 and ES-0009-50, the whole test is then for research use only! |


 PRODUCTS
PRODUCTS